Myriad Genetics Receives FDA Approval for Breast Cancer Diagnostic Test
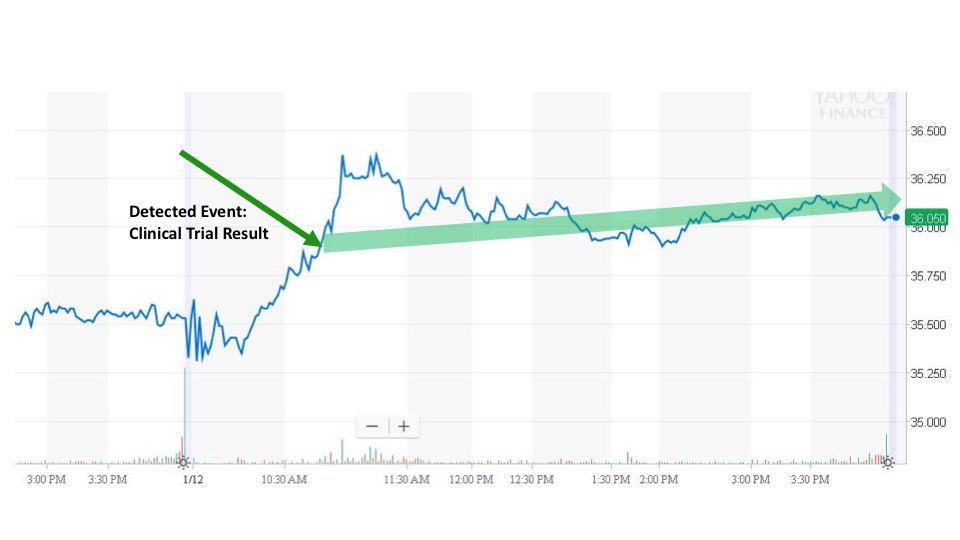
On January 12, Myriad Genetics, Inc. (MYGN) announced that the FDA has approved BRACAnalysis CDx as a companion diagnostic for metastatic breast cancer. Healthcare professionals will be able to use this to identify patients with HER2-negative metastatic breast cancer who have a BRCA mutation and may be candidates for treatment with the PARP inhibitor, Lynparza. Myriad is a leader in molecular diagnostics, and this is the first and only test approved by the FDA for use in this treatment diagnostic tool.
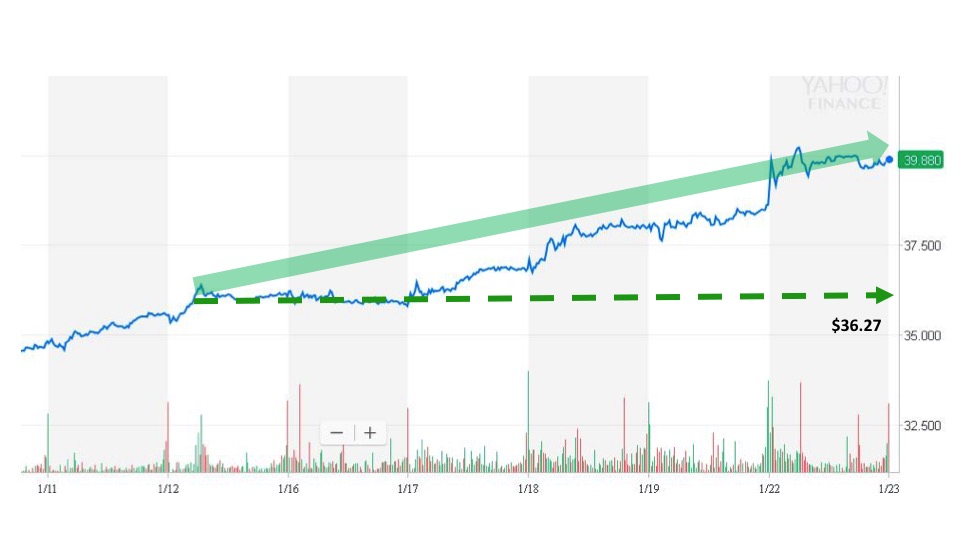
Sonal received the news and issued an alert at 11:16 am. The next trade occurred at 11:17 am for $36.27. The stock price held above $36 for the rest of the day before closing at $36.05.
The stock price climbed higher during the five trading days that followed the announcement. Myriad closed at $39.88 on January 22, which marks a gain of 10% in the days after the event.
If you want to learn more about trading on clinical trial results, visit the Knowledge Center.
To see the latest weekly webinar, you can visit our Live Webinar page.
Subscribe here if you would like to start receiving these signals in real-time and start trading!