Sorrento Therapeutics Subsidiary Receives FDA Approval for Non-Opiod Ztlido
On February 28, Sorrento Therapeutics (SRNE) Subsidiary, Scilex, received FDA approval for non-opiod Ztlido. The drug is a lidocaine topical system used to treat pain. Sorrento hopes to have it commercially available to patients later this year.
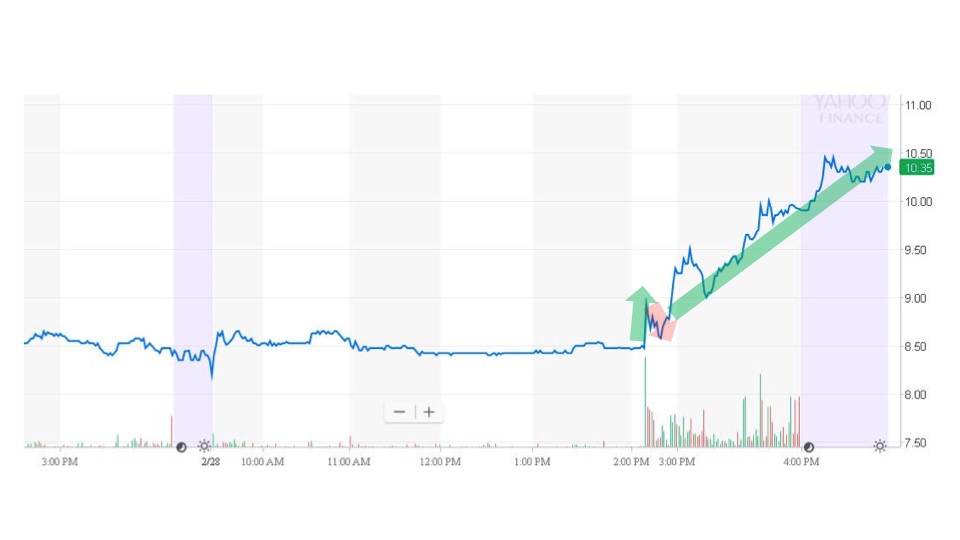
Sonal received the news and sent an alert to subscribers at 2:11 pm. The next trade took place for $8.95 at 2:45 pm. After a brief fade, the price pushed higher until the close at $9.95.
If you want to learn more about trading on clinical trial results, visit the Knowledge Center.
To see the latest weekly webinar, you can visit our Live Webinar page.
Subscribe here if you would like to start receiving these signals in real-time and start trading!