Reata Pharmaceuticals Reports Positive Phase 2 Results From Two Clinical Trials
On July 23, Reata Pharmaceuticals, Inc. (RETA) announced results from two Phase 2 clinical trials of bardoxolone in patients with chronic kidney disease. The one-year results for the Phase 2 portion of CARDINAL, a study of bardoxolone in patients with chronic kidney disease due to Alport syndrome, found that patients had no drug-related negative side effects and had significantly higher estimated glomerular filtration rates (eGFR). Reata also reported final results for the Phase 2 autosomal dominant polycystic kidney disease (ADPKD) cohort of PHOENIX. Patients had no drug-related negative side effects and had significantly higher estimated glomerular filtration rates (eGFR).
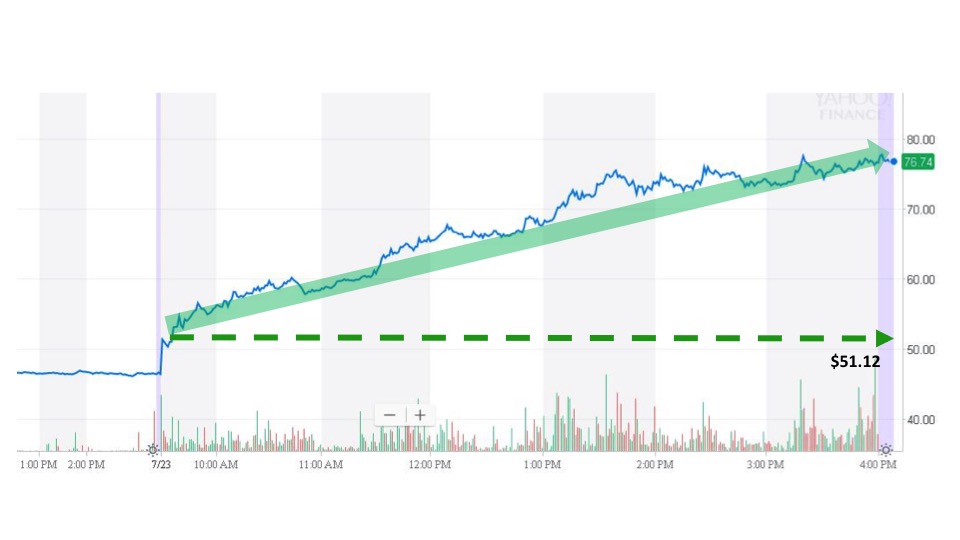
Sonal received the news and sent an alert at 6:45 am. The next trade took place at the market open for $51.12. Reata’s stock price soared throughout the day before closing at $76.55 for a gain of 49.75% on the event day.
If you want to learn more about trading on clinical trial results, visit the Knowledge Center.
To see the latest weekly webinar, you can visit our Live Webinar page.
Subscribe here if you would like to start receiving these signals in real-time and start trading!