Lannett Announces FDA Acceptance of New Drug Application
On December 1, Lannett Company, Inc. (LCI) announced FDA acceptance of its 505(b)(2) New Drug Application (NDA) for Cocaine Hydrochloride (HCl) Topical Solution. The drug is a proprietary local topical anesthetic with the proposed name Numbrino. The submission is supported by two successful Phase 3 clinical trials.
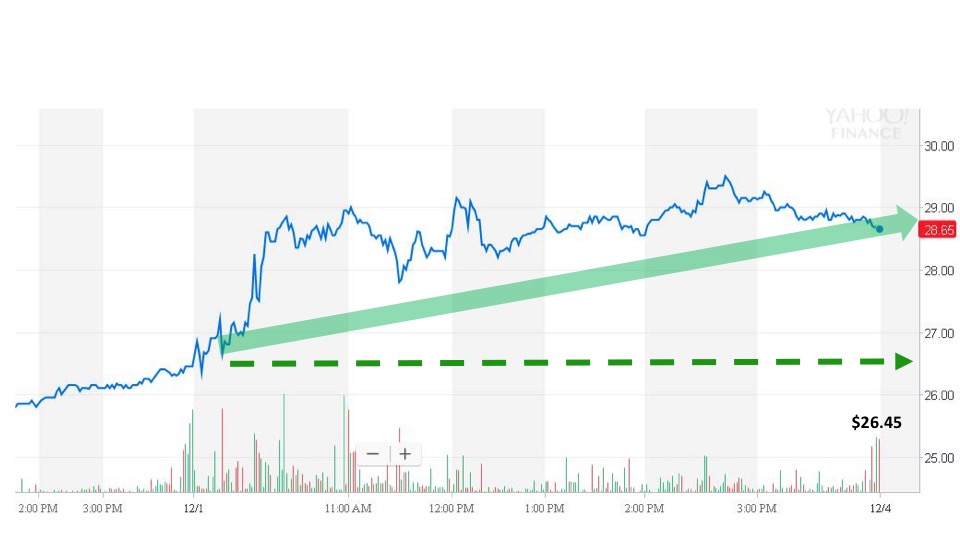
Sonal detected the event and sent an alert to subscribers at 6:53 am. The next trade occurred at 8:04 am for $26.45. The stock opened for trading during the regular market session at $26.55.
Lannett’s stock price continued to climb throughout the day on the positive news before closing for the day at $28.65. That makes a gain of 8.3% for the day following the announcement.
If you want to learn more about trading on clinical trial results, visit the Knowledge Center.
To see the latest weekly webinar, you can visit our Live Webinar page.
Subscribe here if you would like to start receiving these signals in real-time and start trading!